Caltech condensed-matter theorist Gil Refael explained his scientific raison dê’tre early in my grad-school career: “What really gets me going is seeing a plot [of experimental data] and being able to say, ‘I can explain that.’” The quote has stuck with me almost word for word. When I heard it, I was working deep in abstract quantum information theory and thermodynamics, proving theorems about thought experiments. Embedding myself in pure ideas has always held an aura of romance for me, so I nodded along without seconding Gil’s view.
Roughly nine years later, I concede his point.
The revelation walloped me last month, as I was polishing a paper with experimental collaborators. Members of the Institute for Quantum Optics and Quantum Information (IQOQI) in Innsbruck, Austria—Florian Kranzl, Manoj Joshi, and Christian Roos—had performed an experiment in trapped-ion guru Rainer Blatt’s lab. Their work realized an experimental proposal that I’d designed with fellow theorists near the beginning of my postdoc stint. We aimed to observe signatures of particularly quantum thermalization
Throughout the universe, small systems exchange stuff with their environments. For instance, the Earth exchanges heat and light with the rest of the solar system. After exchanging stuff for long enough, the small system equilibrates with the environment: Large-scale properties of the small system (such as its volume and energy) remain fairly constant; and as much stuff enters the small system as leaves, on average. The Earth remains far from equilibrium, which is why we aren’t dead yet.&
In many cases, in equilibrium, the small system shares properties of the environment, such as the environment’s temperature. In these cases, we say that the small system has thermalized and, if it’s quantum, has reached a thermal state.
The stuff exchanged can consist of energy, particles, electric charge, and more. Unlike classical planets, quantum systems can exchange things that participate in quantum uncertainty relations (experts: that fail to commute). Quantum uncertainty mucks up derivations of the thermal state’s mathematical form. Some of us quantum thermodynamicists discovered the mucking up—and identified exchanges of quantum-uncertain things as particularly nonclassical thermodynamics—only a few years ago. We reworked conventional thermodynamic arguments to accommodate this quantum uncertainty. The small system, we concluded, likely equilibrates to near a thermal state whose mathematical form depends on the quantum-uncertain stuff—what we termed a non-Abelian thermal state. I wanted to see this equilibration in the lab. So I proposed an experiment with theory collaborators; and Manoj, Florian, and Christian took a risk on us.
The experimentalists arrayed between six and fifteen ions in a line. Two ions formed the small system, and the rest formed the quantum environment. The ions exchanged the -,
-, and
-components of their spin angular momentum—stuff that participates in quantum uncertainty relations. The ions began with a fairly well-defined amount of each spin component, as described in another blog post. The ions exchanged stuff for a while, and then the experimentalists measured the small system’s quantum state.
The small system equilibrated to near the non-Abelian thermal state, we found. No conventional thermal state modeled the results as accurately. Score!
My postdoc and numerical-simulation wizard Aleks Lasek modeled the experiment on his computer. The small system, he found, remained farther from the non-Abelian thermal state in his simulation than in the experiment. Aleks plotted the small system’s distance to the non-Abelian thermal state against the ion chain’s length. The points produced experimentally sat lower down than the points produced numerically. Why?
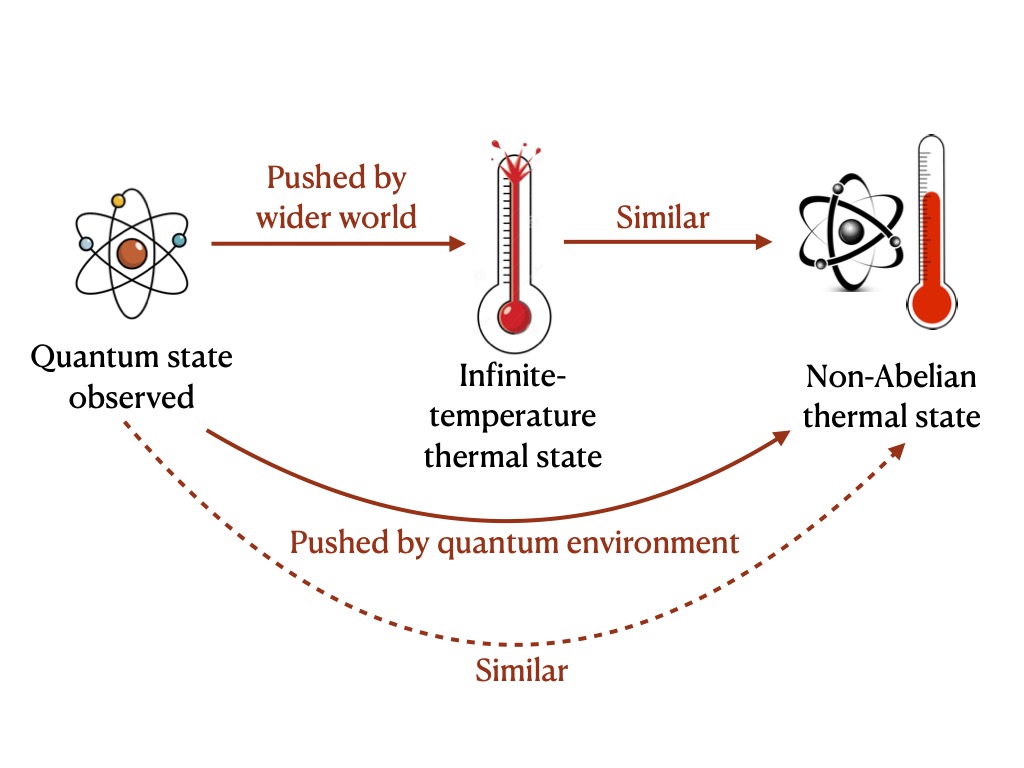
I think I can explain that, I said. The two ions exchange stuff with the rest of the ions, which serve as a quantum environment. But the two ions exchange stuff also with the wider world, such as stray electromagnetic fields. The latter exchanges may push the small system farther toward equilibrium than the extra ions alone do.
Fortunately for the development of my explanatory skills, collaborators prodded me to hone my argument. The wider world, they pointed out, effectively has a very high temperature—an infinite temperature.1 Equilibrating with that environment, the two ions would acquire an infinite temperature themselves. The two ions would approach an infinite-temperature thermal state, which differs from the non-Abelian thermal state we aimed to observe.
Fair, I said. But the extra ions probably have a fairly high temperature themselves. So the non-Abelian thermal state is probably close to the infinite-temperature thermal state. Analogously, if someone cooks goulash similarly to his father, and the father cooks goulash similarly to his grandfather, then the youngest chef cooks goulash similarly to his grandfather. If the wider world pushes the two ions to equilibrate to infinite temperature, then, because the infinite-temperature state lies near the non-Abelian thermal state, the wider world pushes the two ions to equilibrate to near the non-Abelian thermal state.
I plugged numbers into a few equations to check that the extra ions do have a high temperature. (Perhaps I should have done so before proposing the argument above, but my collaborators were kind enough not to call me out.)&
Aleks hammered the nail into the problem’s coffin by incorporating into his simulations the two ions’ interaction with an infinite-temperature wider world. His numerical data points dropped to near the experimental data points. The new plot supported my story.
I can explain that! Aleks’s results buoyed me the whole next day; I found myself smiling at random times throughout the afternoon. Not that I’d explained a grand mystery, like the unexpected hiss heard by Arno Penzias and Robert Wilson when they turned on a powerful antenna in 1964. The hiss turned out to come from the cosmic microwave background (CMB), a collection of photons that fill the visible universe. The CMB provided evidence for the then-controversial Big Bang theory of the universe’s origin. Discovering the CMB earned Penzias and Wilson a Nobel Prize. If the noise caused by the CMB was music to cosmologists’ ears, the noise in our experiment is the quiet wailing of a shy banshee. But it’s our experiment’s noise, and we understand it now.
The experience hasn’t weaned me off the romance of proving theorems about thought experiments. Theorems about thermodynamic quantum uncertainty inspired the experiment that yielded the plot that confused us. But I now second Gil’s sentiment. In the throes of an experiment, “I can explain that” can feel like a battle cry.
1Experts: The wider world effectively has an infinite temperature because (i) the dominant decoherence is dephasing relative to the product eigenbasis and (ii) the experimentalists rotate their qubits often, to simulate a rotationally invariant Hamiltonian evolution. So the qubits effectively undergo dephasing relative to the
,
, and
eigenbases.




Comments